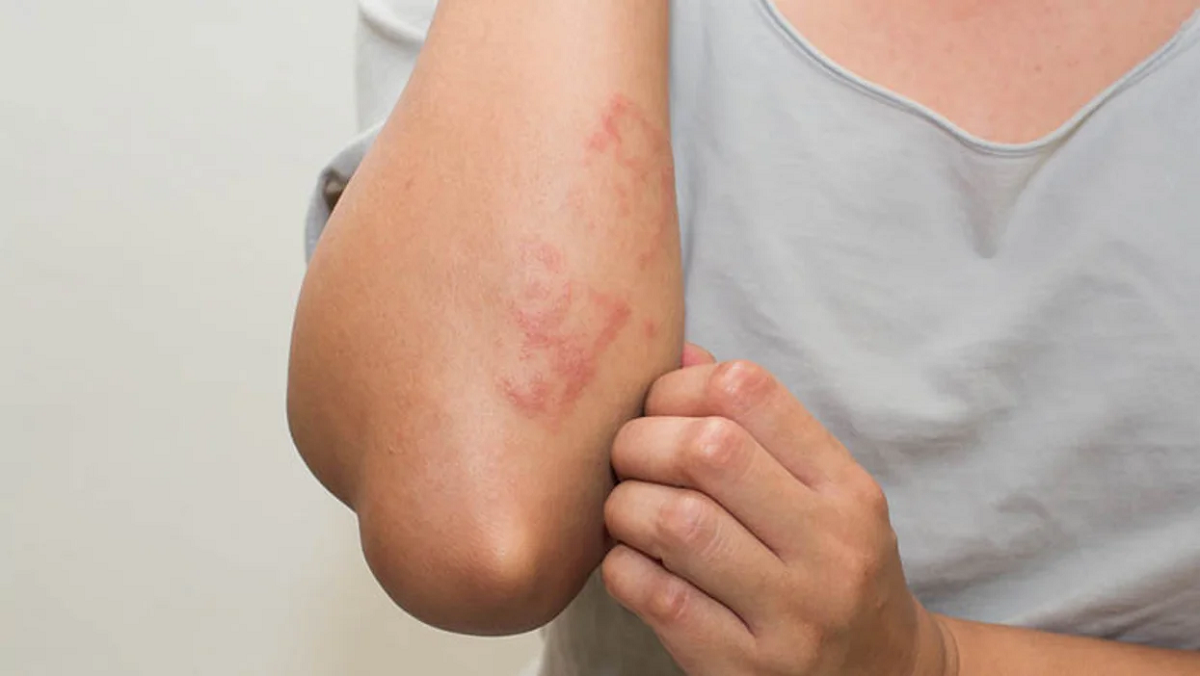
For moderate-to-severe atopic dermatitis, the oral Janus kinase 1 (JAK1) inhibitor abrocitinib at 200 mg or 100 mg once daily results in significantly greater reductions in signs and symptoms versus placebo, according to a study published in the March 25 issue of the New England Journal of Medicine.
Thomas Bieber, M.D., Ph.D., from the University Hospital of Bonn in Germany, and colleagues randomly assigned 838 patients with atopic dermatitis that was unresponsive to topical agents or that warranted systemic therapy to receive 200 mg or 100 mg of abrocitinib orally once daily, 300 mg dupilumab subcutaneously every other week, or placebo in a 2:2:2:1 ratio (226, 238, 243, and 131 patients, respectively).
The researchers found an Investigator’s Global Assessment response at week 12 in 48.4, 36.6, 36.5, and 14.0 percent of patients in the 200-mg abrocitinib group, 100-mg abrocitinib group, dupilumab group, and placebo group, respectively. The corresponding proportions of patients in each group with an Eczema Area and Severity Index-75 response at week 12 were 70.3, 58.7, 58.1, and 27.1 percent. With respect to itch response at week 2, the 200-mg dose, but not the 100-mg dose, of abrocitinib was superior to dupilumab. With respect to most other other key secondary end-point comparisons at week 16, neither abrocitinib dose differed significantly from dupilumab.
“Longer and larger trials are necessary to determine the efficacy and safety of abrocitinib and to compare it with other JAK inhibitors and with biologic agents used for the treatment of atopic dermatitis,” the authors write.
The study was funded by Pfizer, the developer of abrocitinib.
Abstract/Full Text (subscription or payment may be required)




Leave a Reply